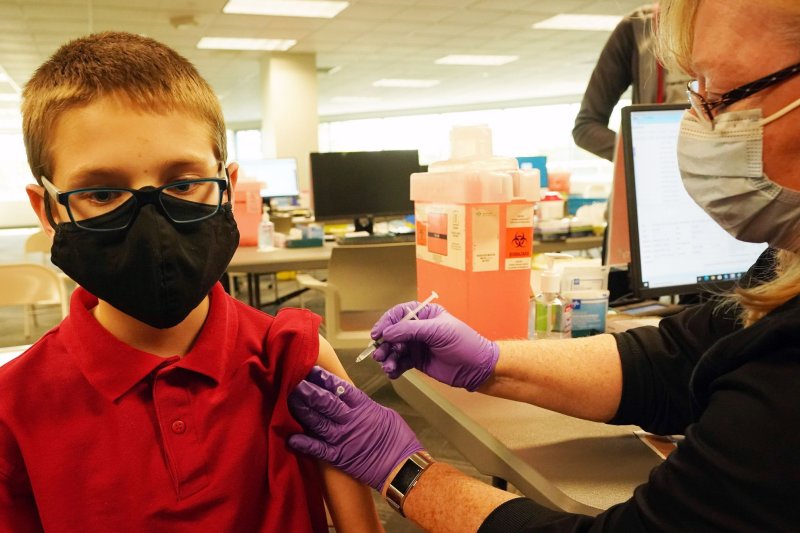
June 8 (UPI) -- Pharma company Pfizer said Tuesday it will begin testing the effectiveness of its COVID-19 vaccine in larger groups of children 11 and younger.
The company said it and partner BioNTech will conduct a study among 4,500 children at dozens of clinical sites in the United States, Poland, Spain and Finland.