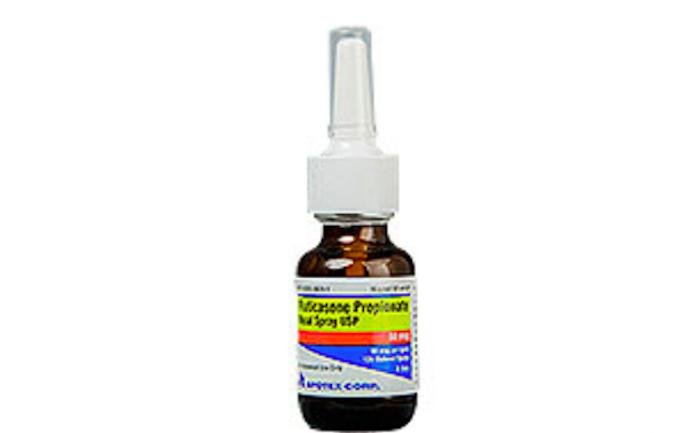
May 31 (UPI) -- Pharmaceutical company Apotex Corp. announced a voluntary recall of its Fluticasone Propionate Nasal Spray on Thursday after it was found to contain glass particles.
The Florida-based company said it was alerted to the small glass particles in its Fluticasone Propionate Nasal Spray USP 50 mcg per spray 120 Metered Sprays through a customer complaint and warned the glass could cause the pump to malfunction and result in user discomfort.