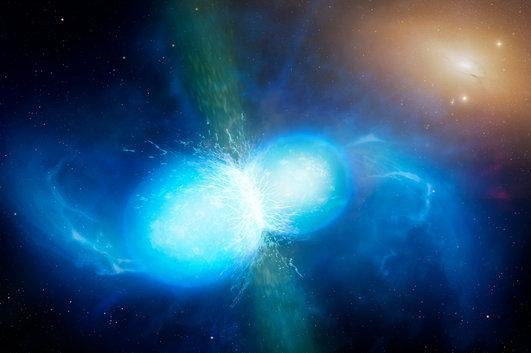
Scientists have identified freshly forged strontium in the spectra of a neutron-star merger. Photo by
University of Warwick/Mark Garlick/ESO
Oct. 23 (UPI) -- Scientists have for the first time identified a freshly forged heavy metal element inside a neutron star merger.
The element, strontium, was found in the spectra emanating from the neutron star merger GW170817. Scientists detailed the discovery in a paper published Wednesday in the journal Nature.
Gravitational wave machines first picked up the signal produced by GW170817 in 2017. Using the European Southern Observatory's Very Large Telescope, scientists traced the signal to its origin and imaged the radiation produced by a pair of merging neutron stars.
The explosion produced by the merger, kilonova AT2017gfo, was the first for which detailed spectra were captured. Within the spectral lines captured by VLT, scientists identified the signature of strontium. Researchers also found evidence that the heavy metal was produced via the r-process, or rapid neutron capture.
Scientists have struggled to spot elements in the atmospheres of nearby exoplanets, but were able to pick out the creation of strontium deep inside a galaxy located 130 million light years away.
"Exoplanets are not very bright, so they rely on the light of the star they orbit to shine through the very small atmosphere of the planet," Darach Watson, researcher at the University of Copenhagen, told UPI. "In this case, the kilonova is a massive expanding fireball of radioactive elements the size of the entire solar system and is 500 million times brighter than the sun."
Hydrogen and helium were produced in abundance by the Big Bang, and elements in the mass range from helium to iron are forged by stellar fusion and supernovas. But until now, scientists had failed observe the phenomena that yield heavier elements.
When researchers first studied the kilonova spectra in 2017, they failed to spot the newly minted strontium. This go around, Watson and his researcher partners used a new analysis strategy.
"We took a different, less theoretical and more phenomenological approach to the data," he said. "We first realized that the spectrum we observed was very similar to a simple thermal spectrum, a blackbody spectrum. When we did that, we could subtract the thermal spectrum and see two major features, which we then had to identify."
Sophisticated analysis confirmed that only strontium produced by the r-process could properly account for the two major features -- the positions and strength of the absorption features in the spectra.
In addition to offering new details on how heavy elements are forged, the latest research also offered fresh insights into the nature of neutron star mergers. Scientists were able to deduce that the kilonova's outer layers are spherical and contain mostly lighter heavy elements. Researchers also confirmed that the merger produced such extreme densities that ghostly particles called neutrinos, at least for a moment, can't escape.
"It's quite exciting that we've seen strontium, because it's among the lightest of the heavy elements -- recall that there are many heavy elements, about 60 -- and that requires a very large amount of neutrinos in the merger to help break down the neutrons that would otherwise have made elements heavier than strontium," Watson said.
Scientists have known theoretically how all of the elements are created for several decades. Now, with the observation of strontium, freshly forged by the r-process, they're a bit closer to nailing down exactly how these theoretical processes play out in the cosmos. But there are still a few elemental sources to track down.
"We are keen to be able to see any new-created lanthanide element, or elements in the platinum group such as iridium, platinum, gold, or even higher mass than this, such as uranium," Watson said. "This is because they lie at increasingly high atomic masses and require different physical conditions, such as a suppression of the neutrinos, and for some atoms, may require exotic ultra-heavy atoms to radioactively decay down to these heavier elements."