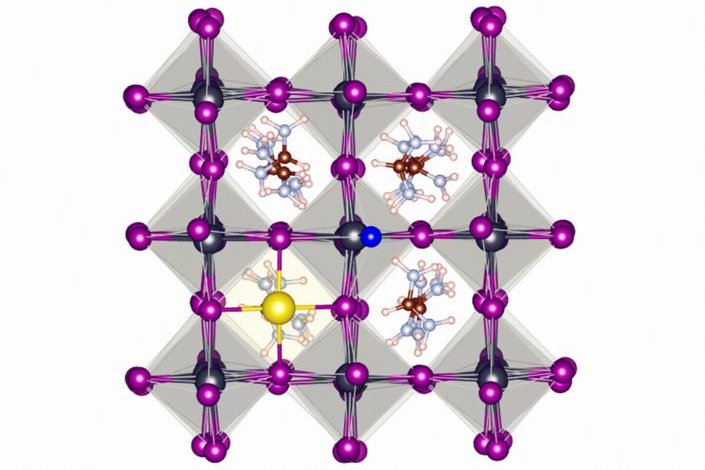
Fluoride stablizes perovskite solar cells by encouraging the formation of strong hydrogen bonds and ionic bonds on the surface of the perovskite material. Photo by Eindhoven University of Technology
May 13 (UPI) -- The addition of a small amount of fluoride, the same chemical added to municipal water to prevent tooth decay, increases the stability of perovskite solar cells.
Perovskite is the cheapest solar cell material available, and its efficiency has been rapidly improving. Unfortunately, it's not all that durable. Perovskite degrades quickly, undermining its long-term reliability.
Over time, solar panels made of perovskite get significantly less efficient at converting the sun's rays into energy. The economic advantages of the material's affordability are quickly eroded.
Researchers at the Eindhoven University of Technology in the Netherlands found they can solve the problem of perovskite's fragility by adding a little bit of fluoride during the production process. They described their work this week in the journal Nature Energy.
Just as the chemical helps protect tooth enamel, scientists found the fluoride ions form a protective layer around perovskite crystal, preventing the ill effects of light, heat and moisture.
"Our work has improved the stability of perovskite solar cells considerably," Shuxia Tao, assistant professor at Eindhoven University of Technology's Center for Computational Energy Research, said in a news release. "Our cells maintain 90 percent of their efficiency after 1,000 hours under extreme light and heat conditions. This is many times as long as traditional perovskite compounds. We achieve an efficiency of 21.3 percent, which is a very good starting point for further efficiency gains."
The fluoride encouraged the formation of strong hydrogen and ionic bonds on the surface of perovskite lattice. Simulations showed the electronegativity of fluoride ions is responsible for its ability to form strong bonds with neighboring elements.
Scientists plan to continue tweaking their process for adding fluoride to the process of making perovskite solar cells. Their goal is to create fluoride-enhanced perovskite solar cells that maintain 85 percent of their original efficiency after ten to fifteen years.
"We expect it will take another five to ten years for these cells to become a commercially viable product. Not only do we need to further improve their efficiency and stability, we also need to gain a better theoretical understanding of the relevant mechanisms at the atomic scale," said Tao. "We still don't have all the answers to why some materials are more effective than others in increasing the long-term stability of these cells."