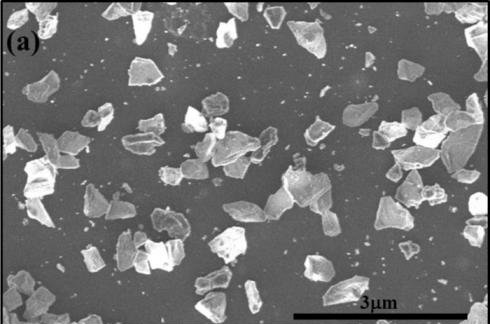
A mixture of nanodiamond and microdiamond Q-carbon created using the new technology. Photo by AIP/ALP Materials
RALEIGH, N.C., Nov. 30 (UPI) -- Most diamonds are made under unique conditions -- natural or man-made -- involving extreme heat and pressure. But researchers at North Carolina State University have discovered a new diamond-like phase of carbon that can be attained at room temperature.
"We've now created a third solid phase of carbon," Jay Narayan, a material scientists at NC State, said in a press release. "The only place it may be found in the natural world would be possibly in the core of some planets."
Researchers call the new phase Q-carbon. To make it, scientists coated a substrate of sapphire, glass or a plastic polymer with a layer of non-crystalline amorphous carbon. The coated substrate is then blasted with single laser pulse. The pulse lasts just 200 nanoseconds, momentarily raising the carbon's temperature to 4,000 Kelvin before quickly cooling. The surrounding air pressure remains the same.
The result is a uniquely crystalline material, harder than a real diamond and boasting a variety of new properties, most of them unstudied and potentially many more undiscovered.
"Q-carbon's strength and low work-function -- its willingness to release electrons -- make it very promising for developing new electronic display technologies," Narayan said.
Scientists can manipulate the Q-carbon creation process -- lengthening the duration of laser pulse, for example -- to alter the end product. The technique can produce a single crystal or other non-diamond shapes.
"We can create diamond nanoneedles or microneedles, nanodots, or large-area diamond films, with applications for drug delivery, industrial processes and for creating high-temperature switches and power electronics," Narayan explained.
The process is relatively inexpensive, but researchers need to further test the material before it can be substituted for diamonds in industrial applications.
"We can make Q-carbon films, and we're learning its properties, but we are still in the early stages of understanding how to manipulate it," Narayan said. "We know a lot about diamond, so we can make diamond nanodots. We don't yet know how to make Q-carbon nanodots or microneedles. That's something we're working on."
The new material is detailed in several new scientific papers, published in the journal APL Materials.