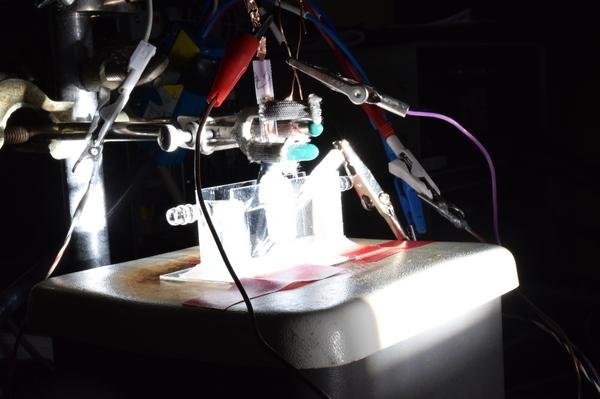
A photoelectrolysis cell splits water into hydrogen and oxygen using a newly developed cheap and efficient catalyst. Photo by Song Jin/University of Wisconsin
MADISON, Wis., Sept. 14 (UPI) -- Many researchers continue to hold hope for an eco-friendly hydrogen economy -- a comprehensive energy-delivery industry based on hydrogen.
Hydrogen can be burned to create heat or used in fuel cells to make electricity. It is also an efficient way to store energy. And unlike fossil fuels, which give off harmful emissions when burned, water is hydrogen's only byproduct.
But like so many other alternative energy panaceas, the technology remains prohibitively expensive. Oxidation-resistant noble metals, like platinum, which are used in water splitting devices, are rare and expensive -- limiting hydrogen's potential as alternative fuel source.
Catalysts are substances which lower the amount of energy necessary to initiate chemical reactions.
Researchers at the University of Wisconsin have discovered a cheap and efficient alternative to the use of noble gases as a catalyst in hydrogen production -- a combination of phosphorus and sulfur (common elements) and cobalt, a metal 1,000 times cheaper than the cheapest noble metals.
Because electricity is currently used to split water and produce hydrogen, some have questioned the true environmental benefits of a hydrogen economy. But engineers have been improving hydrogen-making technologies that use solar energy for water-splitting.
Researchers at Wisconsin say their new catalyst also works with sunlight-powered water-splitting devices.
"We have demonstrated a proof-of-concept device for using this cobalt catalyst and solar energy to drive hydrogen generation, which also has the best reported efficiency for systems that rely only on inexpensive catalysts and materials to convert directly from sunlight to hydrogen," Song Jin, a chemistry professor at Wisconsin, said in a press release.
Jin is the lead author of a new paper on the technology, published this week in the journal Nature Materials.
"If you want to make a dent in the global warming problem, you have to think big," Jin said. "Whether we imagine making hydrogen from electricity, or directly from sunlight, we need square miles of devices to evolve that much hydrogen. And there might not be enough platinum to do that."