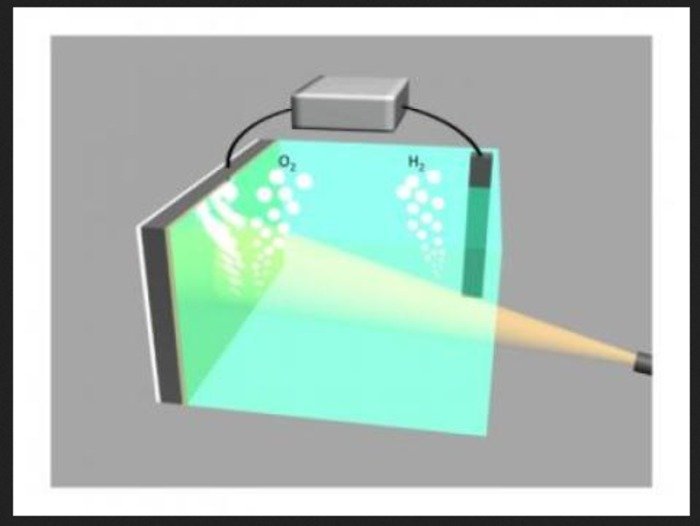
This drawing shows two electrodes splitting water into oxygen (left) and hydrogen (right). The electrodes are connected via an external voltage source. The illuminated silicon electrode (left) is protected from the surrounding electrolyte by a 2-nm film of nickel and uses light energy to assist in the water-splitting process. Credit: Guosong Hong, Stanford University
PALO ALTO, Calif., Nov. 15 (UPI) -- U.S. scientists say a low-cost, long-lasting water splitter made of silicon and nickel could provide hydrogen for fuel cells from light.
Researchers at Stanford University say the new device -- using a silicon semiconductor coated in an ultra-thin layer of nickel -- could supplement solar cells by creating hydrogen for fuel cells that generate electricity at night or when demand is especially high.
"Solar cells only work when the sun is shining," chemistry Professor Hongjie Dai said. "When there's no sunlight, utilities often have to rely on electricity from conventional power plants that run on coal or natural gas."
Using hydrogen-powered fuel cells would be a greener solution, the researchers said.
To produce clean hydrogen for fuel cells, scientists have turned to an emerging technology called water splitting, using two semiconducting electrodes in water that absorb light and use the energy to split the water into its basic components, oxygen and hydrogen.
But finding a cheap way to split water has been a major challenge, the scientists said, and the search is on for inexpensive materials to build water splitters efficient enough to be of practical use.
Electrodes of silicone coated with nickel are one possibility being pursued, they said.
"Nickel is corrosion-resistant," said Stanford graduate student Michael J. Kenney, a co-author of the study published in the journal Science. "It's also an active oxygen-producing catalyst, and it's earth-abundant. That makes it very attractive for this type of application."
The result, they said, is a low-cost, corrosion-free water splitter that shows promise as a green backup for solar cells.