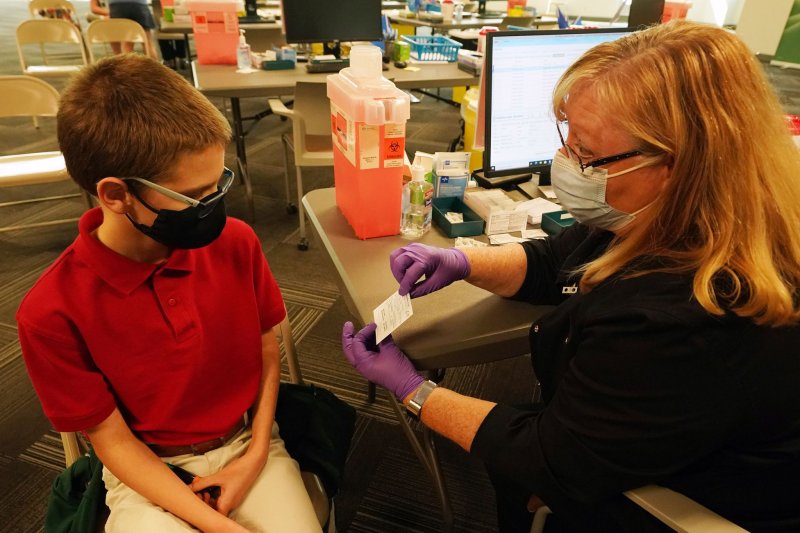
Nurse Kathy Farrar presents Brendan Goldsborough with his vaccination card before receiving his first Covid-19 vaccine in Missouri. Photo by Bill Greenblatt/UPI |
License Photo
June 4 (UPI) -- The rate of COVID-19 hospitalization in adolescents and teens rose in March and April, the U.S. Centers for Disease Control and Prevention reported Friday in its Morbidity and Mortality Weekly Report.
While the rate of adolescents and teens being hospitalized with the coronavirus in the United States had peaked and dipped in January and February this year, the rate more than doubled from mid-March to April, according to CDC researchers.
"I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation," CDC director Dr. Rochelle P. Walensky said Friday in a statement.
Hospitalization rates peaked at 2.1 per 100,000 in early January 2021, then declined to 0.6 in mid-March before rising to 1.3 in April.
Among hospitalized adolescents, nearly one-third required intensive care unit admissions and 5% required invasive mechanical ventilation.
Of the hospitalized adolescents and teens, nearly 30% had no underlying conditions.
"Much of this suffering can be prevented," Walensky said, noting that adolescents and teens older than age 12 are now eligible to receive the COVID-19 vaccine.
Among children aged 12 and older, 60.3% have received at least one dose and 48.8% are fully vaccinated, with most getting the Pfizer-BioNTech vaccine.
In May, Pfizer asked for full federal approval of its vaccine, which would allow it to market the shot directly to consumers.
The vaccine, developed by Pfizer and BioNTech, received emergency use approval for use in people age 16 years and older from the Food and Drug Administration in December. In May, the FDA expanded Pfizer's EUA to include adolescents and teens age 12 to 15.
The full authorization is expected to come in states looking to vaccinate children ahead of the summer and 2021-2022 academic school year.
Moderna said in May that it's vaccine was nearly 96% effective in adolescents and teens, and that it plans to submit an application for emergency use approval this month.
As of Friday, 50.9% of US adults received at least one Covid-19 vaccination dose.
January 31, 2020
National Institutes of Health official Dr. Anthony Fauci (C) speaks about the coronavirus during a press briefing at the White House in Washington, D.C. Health and Human Services Secretary Alexander Azar (L) announced that the United States is declaring the virus a public health emergency and issued a federal quarantine order of 14 days for 195 Americans. Photo by Leigh Vogel/UPI |
License Photo