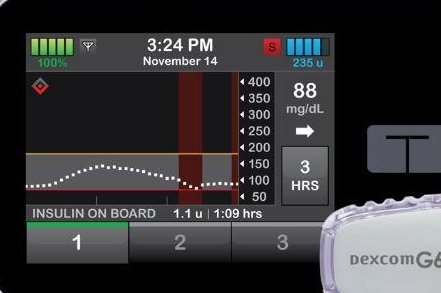
The Tandem Diabetes Care t-Slim X2 insulin pump, which allows a patient to customize treatment, has been approved by the U.S. Food and Drug Administration.
"Diabetes is a complicated disease that requires close monitoring and carefully tailored treatments. We've heard from the patient community that having the ability to customize their own diabetes management devices is important to them," FDA Commissioner Dr. Scott Gottlieb said in an agency news release. "Advances in digital health make more tailored approaches to diabetes care possible."