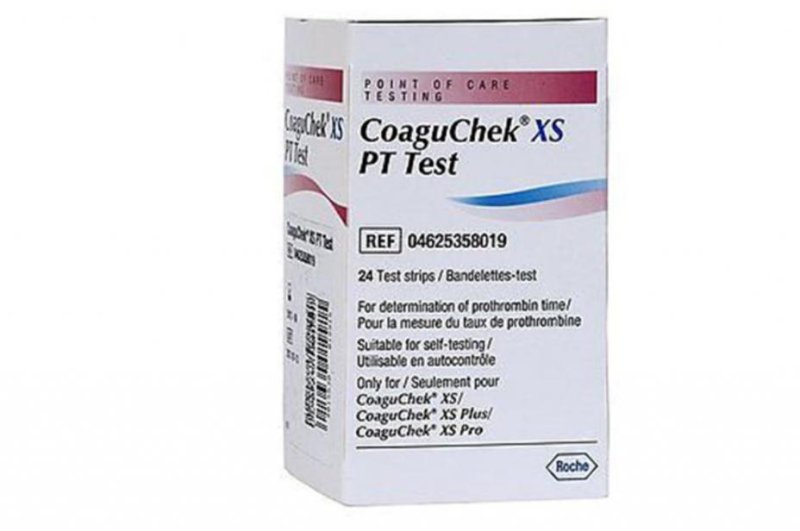
Certain test strips used with Roche meters for monitoring warfarin, a blood thinner prescribed to prevent and treat blood clots, may give inaccurate results. Photo courtesy of
FDA
Nov. 2 (UPI) -- The U.S. Food and Drug Administration has recalled test strips for levels of the blood thinner warfarin because they may provide inaccurate results.
The federal agency said in an advisory on Thursday that Roche Diagnostics' at-home or office medical devices should not be relied upon to adjust drug usage. The Class I recall is the FDA's most serious designation, meaning these devices may cause serious injuries or death.
Rather than relying on these test strips, patients should use an alternative meter device, or blood drawn from a vein and have levels measured by a laboratory test, the FDA said.
The recall involves more than 1.1 million packages of CoaguChek XS PT Test Strips distributed nationwide from Jan. 12 through Monday. The test strips are used with these devices: CoaguChek XS plus, CoaguChek XS Pro, CoaguChek XS professional, CoaguChek XS PST and CoaguChek Vantus. The affected lots numbers are included in the advisory.
The company said it plans to provide new batches of re-calibrated test strips to customers by the end of November.
"These strips are widely used and we are working diligently to warn health care providers and the public about the dangers associated with this recall," Dr. Jeffrey Shuren, director of the FDA's Center for Devices and Radiological Health, said in a statement. "Using faulty strips can lead to serious errors in medication dosage that could cause serious harm or death in some patients."
Warfarin, which is known by the brand names Coumadin and Jantoven, is used to prevent and treat blood clots for certain types of irregular heartbeats, blood clots in the legs or lungs, or certain medical device implants such as artificial heart valves.
Of particular concern, the FDA said, are individuals at increased risk because of mechanical heart valves, irregular heartbeat or those with a recent blood clot. Problems with the CoaguChek XS PT test strips are not likely to be evident to the patient, the FDA said.
Regular monitoring is required while their blood needs to clot.
Results are provided with fingerstick blood drawn from a lancet using a test meter at home or in a doctor's office similar to devices that test blood glucose levels.
The meter reads the test strip, measures how long it takes the blood to clot and provides a result based on a standardized calculation in the form of the International Normalized Ratio.
Roche Diagnostics notified the FDA of 90 medical device reports and two serious patient injuries involving strokes.
The company said the problem is linked to a recent re-calibration of the test strips to a different international standard from earlier this year. The new tests strips will be distributed based on the previous international standard.
The FDA is urging device users are urged to contact their health care provider to get information about alternative test methods and to address questions regarding their individual testing schedule.