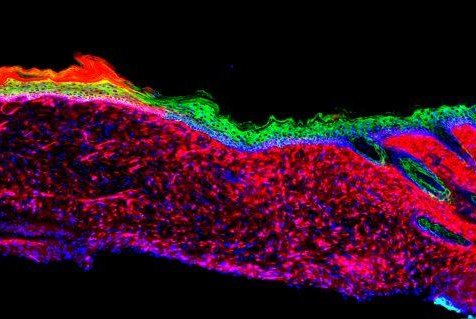
The image represents the successful regeneration of a functional organ -- skin inside a mouse. The skin tissues were generated by converting one cell type, called mesenchymal, which are displayed as red, to basal keratinocyte, which are green. Photo courtesy of
Salk Institute
Sept. 6 (UPI) -- Scientists have developed a technique to convert cells in open wounds into skin cells as an alternative to plastic surgery for treatment of large cutaneous ulcers.
The method involves reprogramming the cells into a stem-cell-like state for healing skin damage, including severe burns, bedsores or chronic diseases such as diabetes. The researchers at the Salk Institute also see this process as a way to counter the effects of aging and better understanding skin cancer.
Their findings were published Wednesday in the journal Nature.
"This knowledge might not only be useful for enhancing skin repair but could also serve to guide in vivo regenerative strategies in other human pathological situations, as well as during aging, in which tissue repair is impaired," senior author Dr. Juan Carlos Izpisua Belmonte, a professor at he Salk Institute, said in a press release.
He said the in vivo regeneration represents an entire three-dimensional tissue like the skin and not just individual cell types as previously shown in research.
Cutaneous ulcers, which can extend through multiple layers of the skin, now are typically treated surgically by transplanting existing skin to cover the wound.
When the ulcer are too large, surgeons often are unable to graft enough skin. An option is a lengthy process of isolating skin stem cells, growing them in the lab and transplanting them back into the patient.
A critical step in wound recovery is the migration -- or transplantation -- of basal keratinocytes into wounds. Although stem-cell-like cells act as precursors to the different skin cells, the large and severe wounds no longer have any basal keratinocytes. As these wounds heal, the multiplying cells are mainly involved in wound closure and inflammation instead of rebuilding healthy skin.
Without taking the cells out of the body, the researchers wanted to directly convert these other cells into basal keratinocytes.
"We set out to make skin where there was no skin to start with," said Masakazu Kurita, a Salk research associate.
By comparing the levels of different proteins of the two cell types -- inflammation and keratinocytes -- they focused on 55 "reprogramming factors" -- proteins and RNA molecules -- that define basal keratinocytes. They narrowed the list to four factors.
In mice with skin ulcers, healthy skin -- known as epithelia -- grew within 18 days.
Three and six months later, the new cells acted like healthy skin cells in a number of molecular, genetic and cellular tests.
"Before going to the clinic, we have to do more studies on the long-term safety of our approach and enhance the efficiency as much as possible," Kurita said.