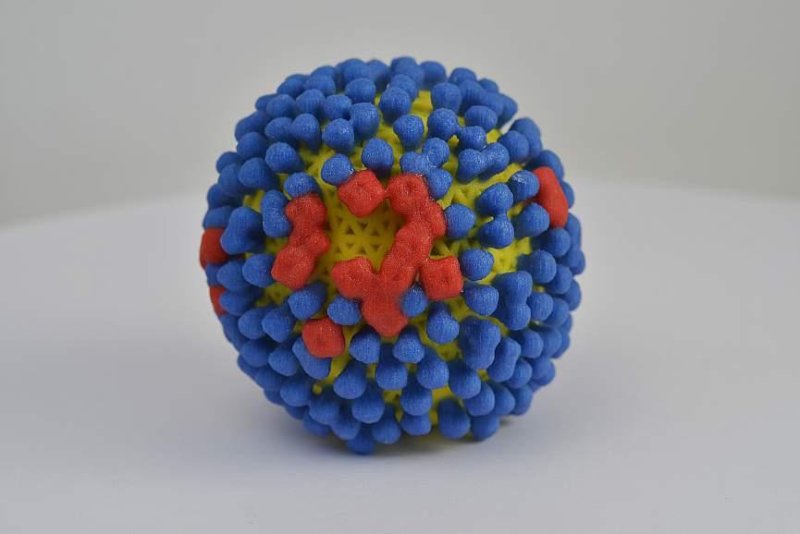
This is a 3D representation of the influenza virus, which is depicted in yellow and covered with proteins called hemagglutinin in blue and neuraminidase red. Photo courtesy of
National Institutes of Health
Sept. 6 (UPI) -- A clinical trial is underway to determine the effectiveness of a topical cream to enhance the immune response to a "pre-pandemic" influenza vaccine.
Investigators at the Baylor College of Medicine in Houston are conducting the trial to see whether the imiquimod cream, which is commonly used to treat genital warts and certain skin cancers, can increase immune response to an H5N1 influenza vaccine.
Fifty participants are being sought for the trial, with the first participant in the study vaccinated on June 19 this year. Researchers hope to have the study completed by next June.
Baylor is among clinical research sites that can rapidly enroll a large number of volunteers to evaluate experimental vaccines against infectious diseases. They are funded and managed by the National Institute of Allergy and Infectious Diseases, which is part of the National Institutes of Health.
"NIAID is pleased to support a clinical trial evaluating an innovative way to boost immune responses to a pre-pandemic vaccine," Dr. Anthony S. Fauci, the NIAID director, said in a press release. "The Vaccine and Treatment Evaluation Units remain a crucial component of our pandemic influenza preparedness efforts."
H5N1 causes severe respiratory illness in birds and, in rare circumstances, humans have contracted the flu through direct or indirect contact with infected birds, such as poultry.
The World Health Organization reported 860 cases of H5N1 influenza, including 454 fatal ones, from 2003 through July 20.
H5N1 influenza doesn't spread easily from human to human but because viruses undergo constant genetic changes, that could change and cause a pandemic, the NIH said.
Participants between 18 and 50 years old will receive an H5N1 vaccine manufactured by Sanofi Pasteur designed for use in a potential pandemic. The vaccine is made from inactivated influenza virus. It was added to the National Pre-pandemic Influenza Vaccine Stockpile in 2007.
Each participant will receive two intradermal doses of the H5N1 vaccine 21 days apart.
In one group, participants will have a cream applied to their upper arm before each vaccination. Aldara is an imiquimod cream manufactured by Valeant Pharmaceutical International, Inc. and approved by the U.S. Food & Drug Administration.
After waiting five to 15 minutes, the vaccine will be administered to the upper arm where the cream was applied.
The control control group will have a placebo aqueous cream applied in the same way before both vaccinations.
Participants must refrain from washing their arm for four to six hours after injection.
They will return to the Houston clinic at regular intervals over seven months to have blood drawn. They also will record any symptoms and medications taken at home.