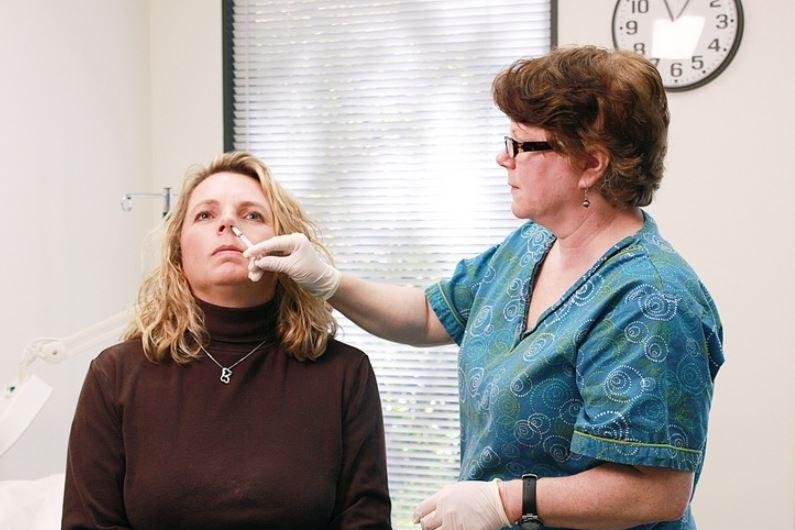
The Centers for Disease Control and Prevention has returned FluMist to its list of recommended flu vaccines, drugmaker AstraZeneca announced. Photo by
Douglas Jordan/CDC
Feb. 22 (UPI) -- FluMist Quadrivalent has returned to the Centers for Disease Control and Prevention's recommended list of flu vaccines, drugmaker AstraZeneca announced.
The CDC's Advisory Committee on Immunization Practices voted Wednesday to recommend FluMist's use after a two-year suspension, the pharmaceutical company said Wednesday.
The only non-injected flu vaccine is back on the recommended list for the 2018-19 flu season, although the strain still needs to be approved by the U.S. Food and Drug Administration.
AstraZeneca's U.S.-based subsidiary, MedImmune, makes FluMist, which is sprayed into the nose to help protect against influenza by children, adolescents and adults ages 2 through 49.
MedImmune said it reformulated the vaccine's H1N1 component after the CDC determined it did not work well against that strain but was effective against others.
Study results of children between 2 and 4 demonstrated that the 2017-2018 H1N1 LAIV post-pandemic strain performed significantly better than the 2015-2016 H1N1 LAIV post-pandemic one and was comparable to vaccines before the 2009 influenza pandemic.
"We are pleased that the ACIP has voted in support of a renewed recommendation for Flumist," Gregory Keenan, AstraZeneca's vice president for U.S. medical affairs, said in a press release. "[We] look forward to continuing to work with public health authorities to optimize protection against influenza."
Limited quantities for the current flu season are available, according to the company.
The vaccine remains on the recommended list in Canada and the European Union.
"The effectiveness of this formulation against [H1N1] is not known, and is likely to remain unknown until the next H1N1 predominant season, presuming adequate uptake of vaccine. We can't predict when this will occur," the CDC's Dr. Lisa Grohskopf told the committee, which approved recommending the vaccine 12-2.
The company said the vaccine spray is not recommended for people with a severe allergy to eggs or to any inactive ingredient in the vaccine; or have ever had a life-threatening reaction to influenza vaccinations.
Also, children under 2 have an increased risk of wheezing after getting the vaccine.