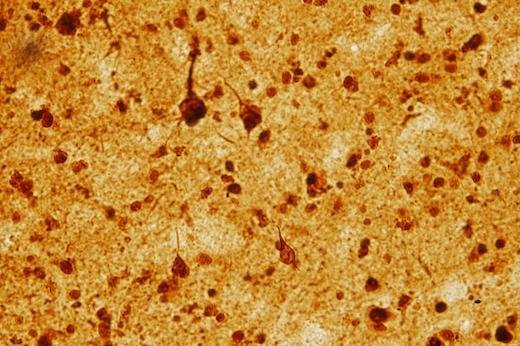
Pictured are LSD1-stained samples from Alzheimer's brain resemble patterns seen with the protein Tau. Researchers at Emory University found that removal of the regulatory gene LSD1 in adult mice induces changes in gene activity similar to Alzheimer's disease. Photo by Emory Alzheimer's Disease Research Center
Oct. 9 (UPI) -- Researchers at Emory University found that removal of the regulatory gene LSD1 in adult mice induces changes in gene activity similar to Alzheimer's disease.
The study, published today in Nature Communications, showed that lysine specific histone demethylase, or LSD1, is agitated in brain samples of people with Alzheimer's disease and frontotemporal dementia, or FTD.
The findings in human patients and mice show that LSD1 plays a central role in neurodegenerative diseases like Alzheimer's and could lead to a possible drug target.
When researchers engineered mice that have LSD1 taken out in adulthood, the mice became paralyzed and cognitively impaired. The mice, however, lacked aggregated proteins in their brains thought to play a significant role in Alzheimer's and FTD.
"In these mice, we are skipping the aggregated proteins, which are usually thought of as the triggers of dementia, and going straight to the downstream effects," Dr. David Katz, assistant professor of cell biology at Emory University School of Medicine, said in a press release.
Researchers examined the patterns of gene activity that were changed in the LSD1-deleted mice, they found signs of inflammation and changes in cell metabolism and signaling similar to those seen in Alzheimer's disease and certain types of FTD.
LSD1's absence appears to release a combination of several stresses that mirror the stresses on brain cells seen in Alzheimer's disease and FTD, representing the first time LSD1 has been linked to neurodegenerative diseases.
"We were amazed to see the accumulation of LSD1 in neurofibrillary tangles in Alzheimer's, and in TDP-43 aggregates in FTD," Dr. Allan Levey, director of Emory's Alzheimer's Disease Research Center said.
"In both diseases, the LSD1 protein was aberrantly localized in the cytoplasm, along with these pathologies. Since LSD1 is normally localized in the nucleus, these findings provided clues to how it might be linked to the massive yet selective neurodegeneration that we observed in the LSD1-deficient mice, in the same cortical and hippocampal regions known to be vulnerable in these two distinct human neurodegenerative diseases."
Researchers say the study may lead to new drug targets for treatment, with compounds that enhance the function of LSD1 or just stop LSD1 from interacting with proteins such as Tau that affect both diseases seen as potentially useful.