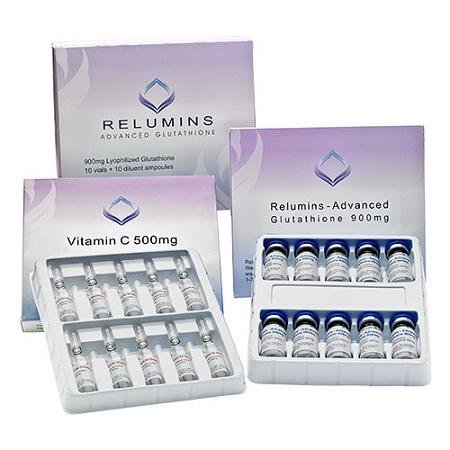
A federal judge has ordered Flawless Beauty LLC, to stop selling and recall its unapproved injectable skin whitening and other beauty products. Photo courtesy of FlawlessBeauty
Sept. 27 (UPI) -- A federal judge has ordered Flawless Beauty LLC, to stop selling and recall its unapproved injectable skin whitening and other beauty products.
The U.S. Department of Justice on Tuesday filed a permanent injunction on behalf of the U.S. Food and Drug Administration alleging that Flawless Beauty sold unapproved and improperly labeled drugs that present serious public health risks, especially its injectable skin whitening products.
The potential risks posed by the injectable skin whitening products include nerve or blood vessel damage, infection, and toxic systemic reactions.
In 2014, U.S. Marshals seized unapproved and improperly labeled drug products by Flawless Beauty, including injectable skin whitening products such as Relumins Advanced Glutathione kits and Tatiomax Glutathione Collagen Whitening kits. Even after the seizure, the FDA said, the company continued marketing and distributing unapproved drugs.
"Despite repeated warnings, Flawless Beauty continued to put patients at risk by selling potentially dangerous and unproven treatments to consumers," Donald D. Ashley, director of the Office of Compliance in the FDA's Center for Drug Evaluation and Research, said in a press release. "We urge consumers to beware of these and other unproven drug products that use deceptive marketing tactics to sell their unsafe products."
Some products sold by Flawless Beauty implied FDA approval or endorsement when they did not have FDA approval.
The consent decree prohibits Flawless Beauty, RDG Imports and its owners from directly or indirectly importing, manufacturing and distributing any drug products until they comply with the Federal Food, Drug, and Cosmetic Act.
The company must also hire an expert to review their drug products and receive written permission from the FDA before resuming distribution of them.