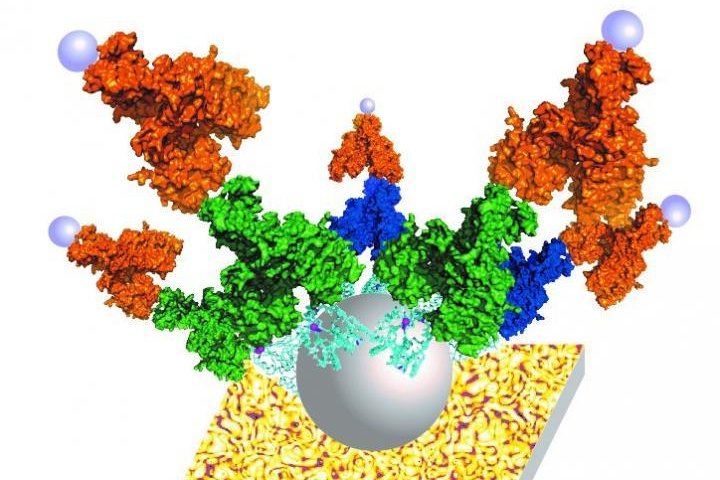
This is an illustration of the ZnT8 protein embedded in a fatty membrane and attached to the P-Gold Assay. Photo by Dax Fu
Sept. 13 (UPI) -- Researchers have developed a new antibody detection technology that could improve the accuracy of diagnostic tests for type 1 diabetes in young children.
Type 1 diabetes, previously known as juvenile diabetes, accounts for 5 percent of all diabetes cases in the United States, is a relatively rare form of diabetes where the pancreas produces no insulin. The National Institutes of Health report rates of type 1 diabetes are increasing by 1.8 percent each year.
A team of researchers from Johns Hopkins University School of Medicine, Stanford University and the University of Florida collaborated on the study that could make population-wide type 1 diabetes screening possible.
"Although current tests are about 94 percent accurate in detecting the antibodies years before children and young adults lose all blood sugar control, they are not accurate enough to rely upon for populationwide screening, so current antibody testing is limited to confirming diagnosis in symptomatic children and adults. Increasing the test accuracy will help expand screening for asymptomatic type 1 diabetes into the general population," Dax Fu, associate professor of physiology at the Johns Hopkins University School of Medicine, said in a news release. "Presymptomatic diagnosis will provide the benefit of beginning preventative therapies."
The study, published today in the Proceedings of the National Academy of Sciences, allows for screening of more autoimmune antibodies in type 1 diabetes than current tests by using a full-length pancreatic protein known as pancreatic zinc transport 8, or ZnT8, that is targeted for autoimmune attack in patients with type 1 diabetes.
The ZnT8 has been known as a major biomarker of type 1 diabetes, however, it has been difficult to efficiently incorporate the entire protein into assays because it loses its shape when removed from pancreatic cells making it unrecognizable to antibodies.
The new technology overcomes that challenge by first inserting the protein into a biomimetric membrane and reconstructing it into its natural shape.
Researchers produced large amounts of ZnT8 by inserting a short sequence of DNA known as plasmid, encoding the gene for ZnT8 into a protein production host derived from human embryonic kidney cells in the laboratory.
The team then isolated the protein from the cells and inserted it into the membrane and then tested the efficacy of the structure for detecting the autoimmune antibodies that recognize ZnT8 in a highly-sensitive assay known as nanostructured, plasmonic near-infrared fluorescence enhancing pGOLD platform.
"The pGOLD-based assay demonstrates superior sensitivity and high-throughput ability with a much lower sample requirement compared to the existing clinical tests," Hao Wan, postdoctoral fellow at Stanford University, said.
Researchers hope the new technology is combined with current tests to reach 99 percent accuracy to start type 1 diabetes screening worldwide.