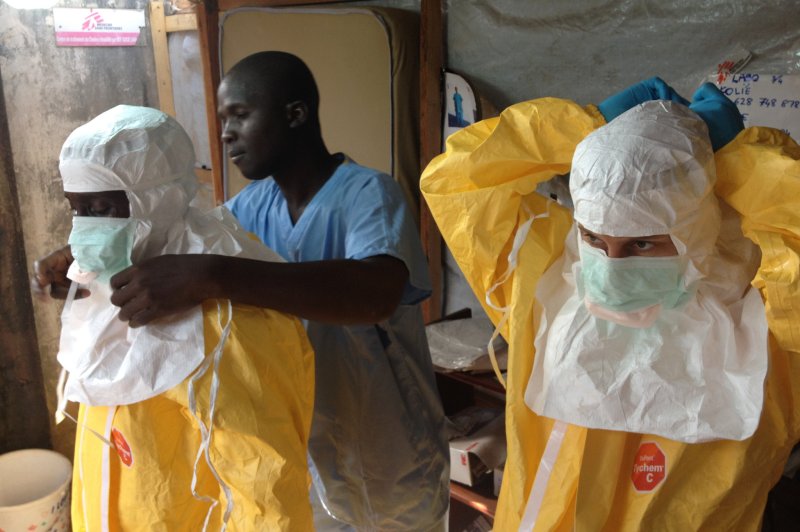
For the first time in West Africa, a case of Ebola was confirmed on 21 March, three weeks after the first alert of a possible viral haemorrhagic fever emerged from Guinea’s Forest region.Though frightening and very lethal, relatively simple precautions can break the cycle of transmission and stop the epidemic from spreading. The European Commission’s Humanitarian Aid and Civil Protection department (ECHO) supports MSF, WHO and IFRC in their efforts to contain the epidemic. UPI/FILE/EC/ECHO/
WASHINGTON, Aug. 28 (UPI) -- As the worsening Ebola epidemic in West Africa claims additional lives each day, world leaders are urgently looking for any sort of medical breakthrough that might reverse the tides and curb the virus' spread. The National Institute of Health will do its part by fast-tracking the first two of several Ebola vaccine candidates for safety trials.
The tests will be headed by the NIH's National Institute of Allergy and Infectious Diseases (NIAID), and will take place at the NIH Clinical Center in Bethesda, Maryland.
The first trials will test the safety of two vaccine candidates -- the NIAID/GSK vaccine, co-developed by NIAID and drug company GlaxoSmithKline, as well as an experimental Ebola vaccine developed by the Public Health Agency of Canada in cooperation with NewLink Genetics Corp. The two Phase 1 trials will see the vaccines tested on a small number of healthy adults. The trials will look for adverse side effects and measure the autoimmune responses elicited in the participants by the vaccines.
The Phase 1 trials are being expedited, as the Ebola outbreak worsens. The World Health Organization reports that since the first Ebola infection was discovered in March of this year, some 1,400 people have perished as a result of the virus. Fatalities have been recorded in Guinea, Liberia, Nigeria, and Sierra Leone.
"There is an urgent need for a protective Ebola vaccine, and it is important to establish that a vaccine is safe and spurs the immune system to react in a way necessary to protect against infection," NIAID Director Anthony S. Fauci said in a press release. "The NIH is playing a key role in accelerating the development and testing of investigational Ebola vaccines."
"Today we know the best way to prevent the spread of Ebola infection is through public health measures, including good infection control practices, isolation, contact tracing, quarantine, and provision of personal protective equipment," Fauci added. "However, a vaccine will ultimately be an important tool in the prevention effort. The launch of Phase 1 Ebola vaccine studies is the first step in a long process."
The two vaccines set to enter Phase 1 testing in the coming days are two of several vaccine candidates being pushed through the safety review process at a hastened pace. Both the vaccines set to now be tested on humans showed promise in previous studies on animals.