New research reveals how components of viper venom disrupt cellular communication in snake bite victims. Photo by Institute of Bioorganic Chemistry of the Russian Academy of Sciences
June 8 (UPI) -- Researchers in Russia have identified one of the complex biochemical mechanisms that give viper venom its deadly potency.
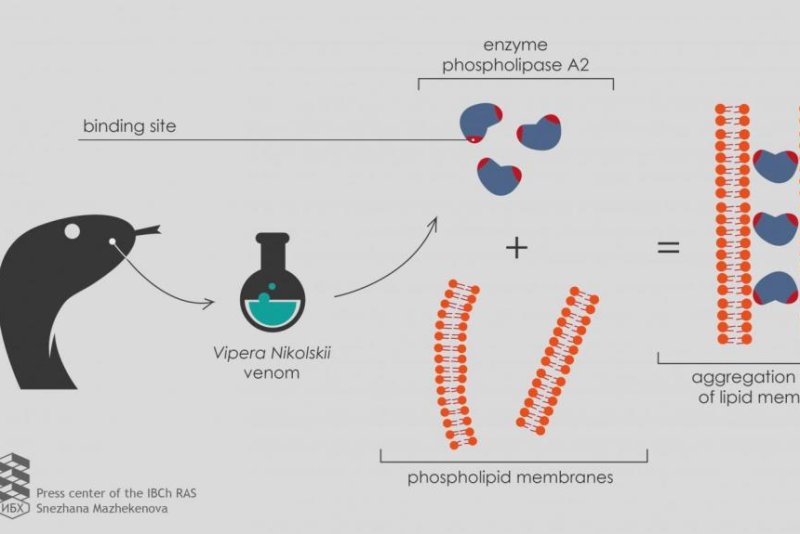
Most venoms use neurotoxins, chemicals that damage nerve tissues. The neurotoxins in viper venom include an enzyme called phospholipase A2, which is of much interest to scientists.
As physicians know, phospholipase A2 is a marker for inflammation in the human body. As inflammation levels rise, phospholipase A2 levels in the blood spike.
For past few years, researchers at the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry RAS have been trying to illuminate and track phospholipase A2 to better understand how the enzyme interacts with the lipid bilayer in cell membranes. In 2016, scientists invented a new fluorescence detection method to measure the energy transfer between two fluorescent dyes in the lipid bilayer.
"To test the new development on as many samples as possible, we turned to the Laboratory of Molecular Toxinology IBCh RAS," Ivan Boldyrev, a senior researcher at the Laboratory of Lipid Chemistry, said in a news release. "The head of the laboratory, Yuri Utkin, has collected a set of phospholipases A2 from poisons of various organisms, including two heterodimeric phospholipases A2 from Vipera nikolskii venom. Each of these enzymes consists of two heterofunctional subunits, polypeptide chains folded in a specific way. However, the toxic effect mechanism of these heterodimers is not clear."
When researchers used the phospholipases A2 from the forest-steppe adder, a viper endemic to Ukraine, eastern Romania, and southwestern Russia, fluorescence failed to occur. Instead, the fluorescent markers decayed.
The viper venom's heterodimeric phospholipases A2 triggered lipid membrane interactions only after scientists supplied the medium with a small negative charge.
"Uncharged membranes with no electric charge on the surface do not combine under the action of heterodimers," said Anna Alekseeva, a junior researcher at the Laboratory of Lipid Chemistry.
"We managed to establish the specificity of heterodimeric phospholipases A2 for negatively charged membranes and determined pH conditions of the medium at which the enzyme manifests itself," added DariaTretyakova, a doctoral student at the Laboratory of Lipid Chemistry.
The new research offers insights into the ways multicomponent snake venoms disrupt biochemical processes in the human body. Scientists detailed their research in the journal Toxicon.