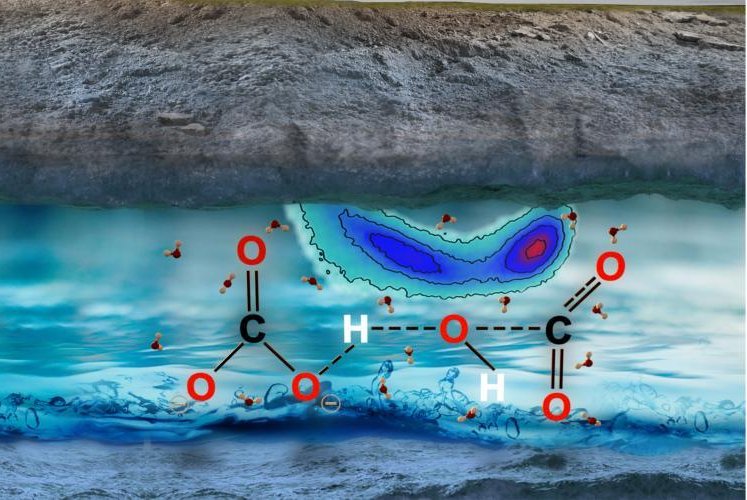
Carbon found in the fluids trapped in Earth’s mantle comes in the form of carbonate and bicarbonate ions, not CO2 molecules. Photo by Giulia Galli, et al./Science Advances
CHICAGO, Nov. 9 (UPI) -- As scientists work to make climate change models more accurate, they must grapple with the myriad ways carbon gets absorbed and released by the planet's biologic, geologic and atmospheric cycles.
Some researchers are trying to understand the intricacies of the carbon cycle deep beneath Earth's surface.
According to a new study published in the journal Science Advances, carbon dissolved in deep-lying, highly pressurized water does not exist as carbon dioxide, but as carbonate and bicarbonate ions.
Scientists say the chemical anomalies can be explained by the extreme conditions found in fissures 410 miles deep.
"Experiments at these extreme conditions remain very difficult, both in execution and interpretation," Giulia Galli, a molecular engineer at the University of Chicago, said in a news release. "Fortunately developments in theory and increases in computing power have recently made it possible to use molecular dynamics simulation to investigate water and carbon at extreme conditions."
The takeaway from the latest experiments are this: carbon behaves much differently deep inside Earth than it does at the surface.
"This is important because the different forms could lead to different kinds of reservoirs of carbon-containing materials at depth in the planet," explained Russell J. Hemley, research professor at George Washington University. "Calculations such as the ones in this study, together with new experiments prompted by these theoretical results, may be key in determining how much carbon is in the planet."
The new research suggests deep-lying water transports carbon in the form of anions, ions with more electrons than protons. Scientists must now grapple with the reality that water found in the pores and cracks of the rocks in Earth's upper mantle contains more ions than previously thought. Models will need to be recalibrated.
"This presence of ions rather than molecules is a game-changer," added Craig E. Manning, a UCLA earth scientist who was not involved in the research.
What effect this will have on broader carbon models isn't yet clear. More work is needed to illuminate the role of deep-lying carbon cycles on larger geochemical systems.